We describe in this edition a single, convenient system for both cloning and site-directed mutagenesis including deletions, base substitutions and base insertions. In-Fusion HD * Cloning Plus is sequence independent, seamless, directional and flexible enough to use with any vector, and it consistently allows over 95% cloning efficiency. Mutagenesis can be carried out by combining the strength of the In-Fusion HD enzyme with inverse PCR.
A variety of single-purpose mutagenesis kits are currently on the market for generating mutations. However, mutagenesis tasks involving deletion, insertion or substitution of a sequence are typically achieved using a PCR-based method. With In-Fusion HD Cloning Plus, there is no requirement for an additional, separate PCR-based mutation kit as both cloning and mutagenesis can be carried out with the same system.
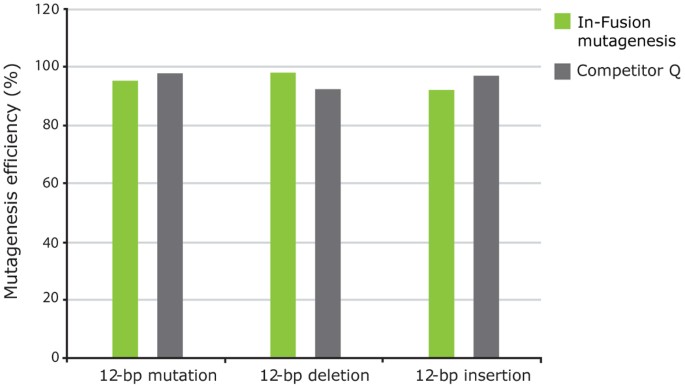
In-Fusion can be used to facilitate mutations with efficiencies comparable to those obtained with single-purpose mutagenesis kits (Fig. 1). Furthermore, this cloning is directional and seamless as no extra bases are added to the target sequence, which is critical when generating mutant constructs.

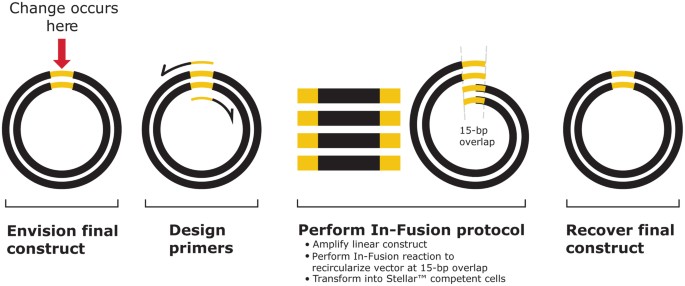
The In-Fusion method makes it easy to perform mutagenesis: it combines the power of the In-Fusion HD enzyme with inverse PCR, a method for rapid in vitro amplification of the DNA sequences that flank a known target sequence region 1 . During inverse PCR, primers are oriented in opposite directions on the target circular cloning vector (Fig. 2). To perform mutagenesis with In-Fusion, the first step involves designing inverse PCR primers that have a 15-bp overlap with each other at their 5′ ends and incorporate the mutation of interest.
Inverse PCR is then performed with the provided high-fidelity CloneAmp ™ HiFi PCR Premix (included in all In-Fusion HD Cloning Plus systems) and the target circular cloning vector. This is followed by the addition of the In-Fusion HD enzyme premix to the linearized target vector PCR product, which is subsequently transformed into the provided highly competent Stellar ™ cells. The next day, colonies are screened for the presence of the mutated target construct; over 95% of correct clones containing the final desired construct are consistently and reproducibly obtained across a broad range of inserts from 0.5 kb to 15 kb in size.
Determine final desired construct. Choose the vector requiring modification and define final, mutated construct (Fig. 2, mutation shown in yellow).
Design inverse PCR primers. Primers should overlap each other by 15 bp at their 5′ ends and incorporate desired mutation (deletion, substitution or insertion). Specific guidelines for mutagenesis primer design are described on the next page.
Combine inverse PCR with In-Fusion. Using an inverse PCR protocol, amplify the target vector with the appropriate inverse PCR primers. Perform the In-Fusion reaction using the inverse PCR product (linearized target vector containing mutation). The linear DNA will recircularize at the site of the 15-bp overlap and will also contain the desired mutagenic changes. Transformation of a portion of the In-Fusion reaction into Stellar ™ competent cells is then performed according to our protocol.
Obtain your final construct. Recover desired mutant from the Stellar ™ cells the following day.
Primer design for deletion mutagenesis
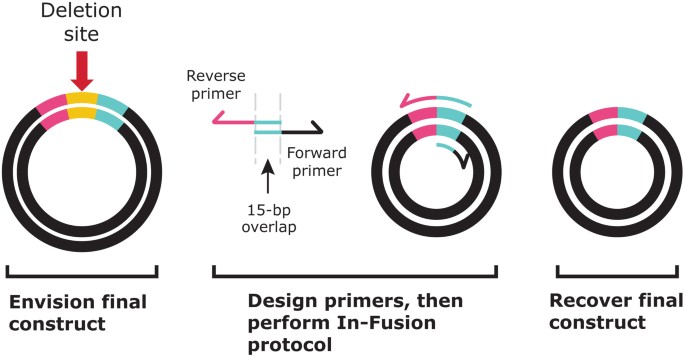
Primer design is a key component of simple, In-Fusion-based deletion mutagenesis. Deleting a region of the target cloning vector requires designing primers with 15-bp overlaps that do not include the bases to be deleted (Fig. 3). For visual interest and easy understanding of the primer design concept, different regions of the vector backbone and primers are marked in color. In Figure 3, the deletion site is marked in yellow and the binding site for the reverse primer (pink and turquoise) spans the deletion. The binding site for the forward primer (turquoise and black) is located on the cloning vector backbone. The common area of turquoise shows the 15-bp primer overlap. There is no gap between the pink and turquoise regions in the actual primer sequence; the deleted nucleotides are not included in either of the primers.
Primer design for base insertions or base substitutions
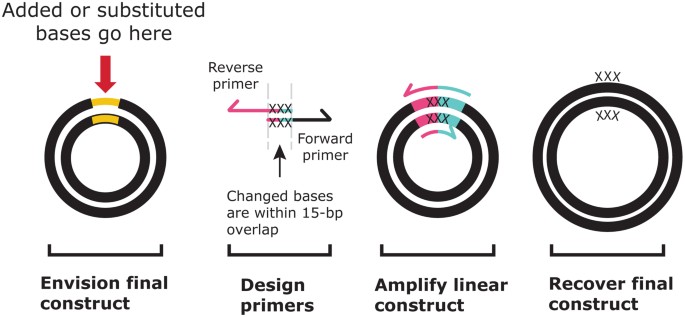
To insert bases, design primers with 15-bp overlaps that contain the desired insertion(s) within the overlapping region (Fig. 4). Only 15 bases are required to overlap, but depending on the length and sequence of the insertion, the overlap may be longer than 15 bp. Additional bases added to the primers will be maintained after the In-Fusion reaction. Similarly, if one or more bases need to be changed within a construct, design primers that include 15-bp overlaps with each other and contain the desired substitutions within the overlapping region (Fig. 4).
Our In-Fusion HD Cloning Plus system can be used for both cloning and mutagenesis, eliminating the need for a separate kit for mutagenesis. It is a fast protocol that combines the strength of the In-Fusion HD enzyme with inverse PCR and consistently achieves over 95% cloning efficiency. In-Fusion allows you to engineer both single and multiple mutations (deletions, insertions and substitutions) quickly and efficiently in one reaction. Finally, a crucial advantage of the In-Fusion system is that it enables the generation of seamless and precisely engineered mutant constructs in which no extra bases are added unless desired by the researcher.